A self-diagnostic test for Hepatitis C infection. Blood sample required.
Take control of your health with our at-home hepatitis C test kit. This convenient and accurate option allows you to test for this common liver infection in the privacy of your own home. The kit comes with everything you need, including a finger-prick blood test and a dropper for transferring a sample to the testing strip. Simply follow the instructions provided and wait for your results, which are available in just minutes. With its high accuracy and reliability, our hepatitis C test kit is an effective option for anyone looking to test for this infection in the comfort of their own home.
The Parsagen Home Hepatitis C Test Kit offers you a discreet method of Hepatitis C testing at your own convenience. This is the exact test kit that is being used in government clinics and test labs. Each pack contains a cassette, micro pipette, alcohol preparation pad, diluent bottle (chemical), lancet (needle) and silicon gel (for keeping contents dry).
Product summary
- Detects hepatitis C surface antigen (HCV).
- High clinical accuracy. Sensitivity: 98.8% specificity: 98.2%.
- For field & health care professional use.
- Tested by certified laboratories worldwide.
- Over 98% accuracy
- TÜV ISO 13485 (ISO’s International Medical Devices Standard) Certified
- GMP (Good Manufacturing Practices) Certified
- Easy to Use
- Total Privacy
Product Description
CATALOG
| Product Name | Specimen | Catalog No. | Quantity per box |
| HCV Strip | Whole Blood/Serum/Plasma | PS-D-0108 | 50T |
| HCV Cassette | Whole Blood/Serum/Plasma | PS-D-0109 | 25T |
KEY POINTS
Detection for: antibody to Hepatitis C Virus in whole blood, serum or plasma;
Specimen volume: Serum or Plasma specimen: 25 μL, or
Venipuncture Whole Blood specimen: 50 μL, or
Fingerstick Whole Blood specimen: 50 μL
Reading time: 10 minutes;
Relative sensitivity: 100.0% (95%CI*: 99.3%~100.0%);
Relative specificity: 99.8% (95%CI*: 99.4%~100%);
Accuracy: 99.9% (95%CI*:99.6%~100.0%).
*Confidence Intervals
INTRODUCTION
Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA virus. HCV is now known to be the major cause of parenterally transmitted non-A, non-B hepatitis. Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis.
INTENDED USE
Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of antibody to Hepatitis C Virus in whole blood, serum or plasma.
For professional in vitro diagnostic use only.
TEST PROCEDURE
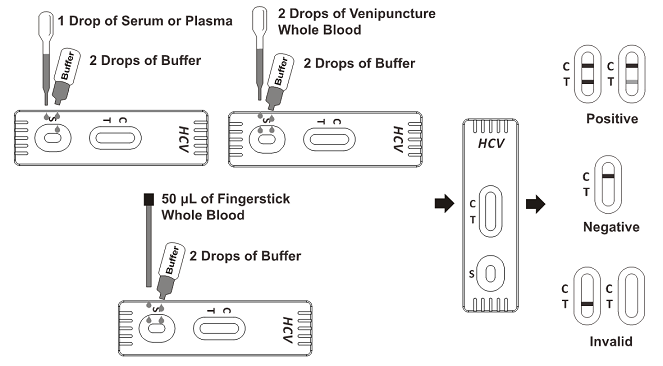
For Cassette:
For Strip:
For Cassette
Bring the pouch to room temperature before opening it. Remove the test cassette from the sealed pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
1. Place the cassette on a clean and level surface.
2. For Serum or Plasma specimen: Hold the dropper vertically and transfer 1 drop of serum or plasma (approximately 25 μL) to the specimen area, then add 2 drops of buffer (approximately 80 μL),and start the timer, see illustration below.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of whole blood (approximately 50 μL) to the specimen area, then add 2 drops of buffer (approximately 80 μL), and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50 μL of fingerstick whole blood specimen to the specimen area of test cassette, then add 2 drops of buffer (approximately 80 μL) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. The test result should be read at 10 minutes. Do not interpret the result after 20 minutes.
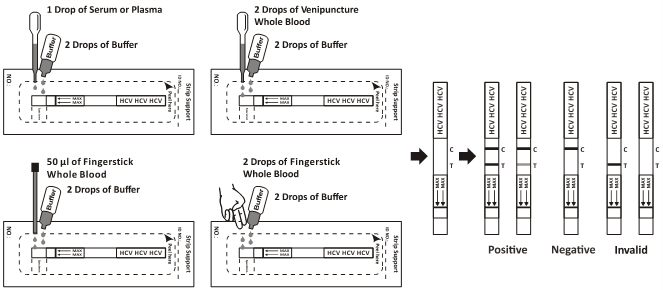
For Strip:
Bring the pouch to room temperature before opening it. Remove the test Strip from the sealed pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
1. Place the Strip on a clean and level surface.
2. For Serum or Plasma specimen: Hold the dropper vertically and transfer 1 drop of serum or plasma (approximately 25μL) to the specimen area,then add 2 drops of buffer (approximately 120 μL),and start the timer, see illustration below.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of whole blood (approximately 50μL) to the specimen area, then add 2 drops of buffer (approximately 120μL), and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50μL of fingerstick whole blood specimen to the specimen area of test strip, then add 2 drops of buffer (approximately 120 μL) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. The test result should be read at 10 minutes. Do not interpret the result after 20 minutes.
PERFORMANCE CHARACTERISTICS
Sensitivity and Specificity
The recombinant antigen used for Parsagen® HCV Cassette (Whole Blood/Serum/Plasma)is encoded by genes for both structural (nucleocapsid) and non-structural proteins. Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) has passed a seroconversion panel and compared with a leading commercial HCV test using clinical specimens.
The results show that the relative sensitivity of Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) is 100%, and the relative specificity is 99.8%.
| Method | Leading commercial HCV test | Total Results | ||
| Parsagen® HCV Cassette | Results | Positive | Negative | |
| Positive | 410 | 3 | 413 | |
| Negative | 0 | 1644 | 1644 | |
| Total Results | 410 | 1647 | 2057 | |
Relative sensitivity: 410/410=100.0% (95%CI*: 99.3%~100.0%);
Relative specificity: 1644/1647=99.8% (95%CI*: 99.4%~100%);
Accuracy: (410+1644)/ (410+0+1644+3) =99.9% (95%CI*:99.6%~100.0%).
*Confidence Intervals
Precision
Intra-Assay
Within-run precision has been determined by using 20 replicates of three specimens: a negative, a HCV low titer positive and a HCV high titer positive. The negative, HCV low titer positive and HCV high titer positive values were correctly identified 100% of the time.
Inter-Assay
Between-run precision has been determined by 20 independent assays on the same three specimens: a negative, a HCV low titer positive and a HCV high titer positive. Three different lots of Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) have been tested over a 3-month period using negative, HCV low titer positive and HCV high titer positive specimens.The specimens were correctly identified 100% of the time.
Cross-reactivity
Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) has been tested by HAMA, RF, HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, Syphilis, HIV, H. Pylori, MONO, CMV, Rubella and TOXO positive specimens. The results showed no cross-reactivity.
Interfering Substances
The following potentially interfering substances were added to HCV negative and positive specimens.
Acetaminophen: 20 mg/dL
Caffeine: 20 mg/dL
Acetylsalicylic Acid: 20 mg/dL
Gentisic Acid: 20 mg/dL
Ascorbic Acid: 2g/dL
Albumin: 2 g/dL
Creatin: 200 mg/dL
Hemoglobin 1000mg/dL
Bilirubin: 1g/dL
Oxalic Acid: 60mg/dL
None of the substances at the concentration tested interfered in the assay.
LIMITATIONS
1. Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) is for in vitro diagnostic use only. This test should be used for the detection of antibodies to HCV in whole blood, serum or plasma specimen.
2. Parsagen® HCV Cassette (Whole Blood/Serum/Plasma) will only indicate the presence of antibodies to HCV in the specimen and should not be used as the sole criteria for the diagnosis of Hepatitis C viral infection.
3. As with all diagnostic tests, all results must be considered with other clinical information available to the physician.
4. If the test result is negative and clinical symptoms persist, additional follow-up testing using other clinical methods is recommended. A negative result at any time does not preclude the possibility of Hepatitis C Virus infection.
Note: The above information is for reference use only. Please refer to the product insert provided with the products before use.
